How Safe Are Our Medicines?
MONITORING THE RISKS OF DRUGS AFTER THEY ARE APPROVED FOR MARKETINGDES Action Canada in collaboration with Women and Health Protection
Medicines have made enormous contributions to health. We are able to treat diseases like meningitis, tuberculosis and syphilis that used to be untreatable. We have powerful painkillers that help to make the last days of a cancer patient's life more bearable. If they are used when needed, medicines can make a difference between life and death, or comfort and pain.
However, all medicines also have side effects. These are effects other than the reason a drug is taken. They range from nuisance effects to serious irreparable harm and even death. Some side effects may be common, others rare.
Some drugs are riskier than others, but none are totally risk-free. That is why medicines should only be used if the expected benefits outweigh possible harm.
| All medicines have side effects. Deciding to take a medicine is a balancing act, weighing possible benefits against possible risks. | |
|
Deaths from medicine use
In 1998, researchers at the University of Toronto published a study looking at how often people experience serious harmful drug reactions and how many people die each year as a result. They looked at U.S. hospital studies over the last 30 years, and only looked at deaths from normal medicine use, not from overdoses or mistakes.
Their results were shocking. They estimated that between 75,000 and 100,000 people die from medicine use each year in the U.S. This would make harmful drug reactions the fourth to sixth leading cause of death. If their higher estimate is correct, only heart disease, cancer and stroke claim more lives. At the same rate in Canada, about 10,000 people would die each year from harmful effects of medicines.
Getting a drug to market in Canada : too much secrecy, too little accountability
To get a drug approved for sale in Canada, the company manufacturing the drug has to test it on cells and tissues, on animals, and finally on people to show that it is acceptably safe and effective.
Health Canada's Health Products and Food Branch then reviews the company's application and decides whether or not the drug can be marketed. If it is approved, the Health Products and Food Branch also approves specific labeling to accompany it. This labeling includes a listing of the health conditions the drug has been shown to treat effectively, warnings about possible harmful effects and about interactions with other medicines, as well as information about who should and who should not use the drug; for example, labeling would indicate whether it can be used safely by children, pregnant women, the elderly, or by people with certain health problems.
In Canada, these decisions are made behind closed doors and neither the public nor physicians have access to the reports of Health Products and Food Branch reviewers or to the information on safety and effectiveness a company submits for review. A company is under no obligation to publish the effectiveness and safety studies submitted for review, and these studies often remain unpublished. Therefore, without access to the reportss submitted to Health Canada, physicians and the public often have very limited information on which to base decisions about whether to try a new drug.
Why is this information kept secret in Canada? The official reason is commercial confidentiality. We don't believe that this is justified. Companies already have patents for their products to protect them from competitors. Public safety should not be a commercial secret.
Freedom of Information legislation is much stronger in the United States than it is Canada. In the U.S., both the unpublished safety and effectiveness information in drug registration files and the assessments of U.S. Food and Drug Administration reviewers are public without causing problems to commercial confidentiality. Furthermore, the U.S. government holds public meetings about drugs that are being considered for approval.
When a drug first comes to market, too little is known
Even if these unpublished studies were to be made available in Canada, our knowledge about the effects of new drugs would be limited. Usually, between 2000 to 3000 people have taken the drug in pre-marketing studies, often only for short periods of time. After it is released on the market, thousands or even millions of people may use the same drug. If 2000 to 3000 people test a drug, serious harmful reactions that occur in less than about 1 in 800 people are unlikely to be discovered.
Furthermore, medicines are often used by people who were not included in pre-marketing studies, such as children or the elderly. They are also often used for longer periods of time. Anti-depressant studies usually last no more than six to eight weeks, whereas these drugs are taken for years at a time. Studies often rely on short-term measures of a drug's effectiveness. These do not always indicate what happens to a person's health in the long run. For example, a study may show that a drug lowers cholesterol or blood pressure, not whether it has any effect on heart disease.
The information companies gather about drug safety in pre-marketing studies is helpful. It just isn't enough. Companies try to get drugs to the market quickly in order to get returns on the investments made in drug development. However, to get a new drug to market in Canada, a company only has to show that it work better than a placebo, or sugar pill. They don't have to compare it to existing treatments. When it comes to medicines, "newer" is not necessarily "better". Between 1991 and 1997, 577 new medicines were approved in Canada. The Patented Medicines Pricing Review Board classified only 8.7% as breakthrough products. Another 41.6 % offered moderate, little or no advantage compared to products that were already available, and 49.7% were only "line extensions" (new dosage forms or other minor modifications).
| THE FIVE-YEAR RULE | |
Because so little is known about possible harmful effects of new medicines, a U.S. non-profit organization, Public Citizen Health Research Group recommends to its members:
|
Pharmaco-vigilance or pharmaco-somnolence?
After they are approved for marketing, drugs are mainly monitored through a system called voluntary spontaneous adverse drug reaction reports. These reports are usually made by physicians. Sometimes pharmacists, nurses or other health professionals also file adverse reaction reports, and rarely do patients file a report themselves.
Physicians can report to the Canadian Adverse Drug Reaction Monitoring Program, in Health Canada's Marketed Health Products Directorate, or to the drug's manufacturer. Companies are required to pass on all serious harmful reactions to Health Canada. A serious reaction is defined as one that caused a person to be hospitalized or stay in the hospital longer, or caused cancer, birth defects, disability or death. This leaves out a many health problems that can affect a person's daily life profoundly.
A 1998 study of more than 500,000 people in the United Kingdom who were using new medicines found that for every 100 serious harmful drug reactions experienced by men, 160 were experienced by women.
"As a simple family doc I have always been concerned that my knowledge in the area of Adverse Drug Reactions was not adequate. Often, when I prescribed a drug and the patient returns with a rash, for example, I don't know if it is a drug reaction or related to the disease I'm treating. Once in a while I have tried to report what I think is an adverse reaction but found it such a cumbersome and time consuming process that I have given up. I bet that there are lots of GP's like me and I expect that the data base for adverse reactions is not very accurate and probably does not reflect the actual occurrence at a clinical level."
A family physician in British Columbia
The tip of the iceberg
In Canada, around 1400 deaths from adverse drug reactions were reported between 1984 and 1994. This is likely to be only about 2% or less of the true number, based on estimates from studies carried out in hospitals. David Kessler, ex-Commissioner of the U.S. Food and Drug Administration, estimates that only about 1% of adverse drug reactions are reported in the U.S.
If as many as 98% of deaths due to adverse drug reactions are not identified as such, what about harmful reactions seen in doctors' offices? A study among family doctors in France compared the number of reported adverse drug reactions during a period of intense monitoring to the number normally reported over the same time period. They found that when doctors were looking for them, they reported suspected serious harmful reactions 4,500 times more often than usual.
Adverse drug reaction reporting catches the tip of the iceberg. It provides an important early warning system, but it isn't enough.
"It makes no more sense to monitor drug safety without knowing the extent of serious injuries than to have a National Highway Transportation Safety Administration without information about automobile accidents or a Federal Aviation Administration without knowing how may airplane crashes have occurred."
T. Moore, B. Patsy and C. Furberg, "Time to Act on Drug Safety," JAMA 1998; 279 (19): 1571-3
What changes is Health Canada making to drug safety monitoring?
Health Canada officials are aware that the current system does not work very well. They have made some changes recently and are recommending more.
The Canadian Adverse Drug Reaction Monitoring Program in Health Canada, responsible for monitoring harmful drug reactions, has started to disseminate information through a newsletter and by making regular reports in the Canadian Medical Association Journal. These reports are a good way to warn physicians of harmful drug effects that have been reported to the Centre or that have been observed in other countries. Unfortunately, they usually do not reach the public.
Health Canada is proposing changes to the approval system that would require companies to submit safety reports for new drugs every six months during the first three years they are licensed. These reports would be more comprehensive than is currently required. However, they would still be based on voluntary adverse drug reaction reports made to the company, so they will still only catch the tip of the iceberg of harmful drug reactions.
In 1997, Health Canada brought in a new type of drug approval, a "Notice of Compliance with Conditions," which requires companies to carry out systematic studies after a drug is approved for marketing. Unfortunately, this type of drug approval was brought in only as a trade-off to bring certain drugs to the market quickly, like AIDS drugs, when less is known about how well they work or how safe they are. Under Canada's secretive approval system, it is also impossible to know what post-marketing studies are required, because this information is considered confidential.
Some of these changes are helpful, others less so. Systematic safety monitoring needs to be brought in for all new drugs, not as a trade-off for weaker regulations, but to improve the standards we have in place now.
How can we improve drug safety monitoring?
RECOMMENDATIONS:
- STRENGTHEN the drug approval system, and only allow new drugs on the market in Canada that show an advantage over existing treatments, whether it is better safety, effectiveness or convenience.
- MAKE the drug approval system a participatory process, and allow full public access to the information on drug safety and effectiveness used by Health Canada, as well as to the reasons why Health Canada decided to accept or refuse a new drug.
- DEVELOP detailed, clear conflict of interest guidelines for scientific, advisory and decision-making committees involved in drug regulation. People with financial ties to the company manufacturing a product should not be involved in decisions about whether that product is approved.
- REQUIRE systematic, scientifically designed follow-up studies once a drug is approved, during the first years it its use, to collect information on its safety when it's used in a large population and under relatively uncontrolled conditions. These post-approval studies should not be a trade-off for weaker drug approvals.
- MAKE adverse drug reaction reporting mandatory for doctors, pharmacists and other health professionals and give it a prominent place in health education.
- USE existing computerized provincial drug prescribing databases for routine, less intense monitoring of health effects of new drug treatments. This type of monitoring should be done in a way that maintains confidentiality and protects privacy.
- HAVE an effective warning system in place to let health professionals and the public know if a problem is suspected, or if a drug has been banned or restricted for safety reasons in another country.
- SET UP special safety studies for drug use in pregnancy and breastfeeding and for drugs used by healthy women over long periods of time. These should be long-term as well as short-term studies.
- IMPLEMENT drug approval processes that allow for the full participation of a wide range of women’s organizations and women’s health activists in the assessment and approval of new products that are used primarily by women, including all new hormonal products for women.
- GIVEN the problem of unnecessary medicalization of women’s lives, a full public review is needed of all drugs currently on the Canadian market that are intended for healthy stages of women’s lives (menstrual cycle, menopause, pregnancy, breastfeeding), for disease prevention in healthy women (for example osteoporosis prevention), or for treatment of mental health problems (anti-anxiety drugs, sleeping pills and antidepressants). This review should involve full participation of women’s organizations, as described above. The aim would be to re-examine licensing decisions and labeling, and to develop policy options to deal with current and future problems related to unnecessary prescribing of drugs to women.
DES Action Canada, in collaboration with Women and Health Protection, published this booklet to raise public awareness about the importance of rigorous safety monitoring following a drug's approval for use.
This publication is the first in a series that examines new debates related to health protection. Health Canada is currently modifying the federal health protection legislation that regulates medicines, food and harmful substances in the environment. The interests of the pharmaceutical and biotechnology industries, the food industry, the chemical industry and the nuclear industry are well represented in Ottawa, while ordinary citizens are virtually excluded from the development of health policies. Health protection for Canadians must be the legislation's first priority.
------------------------------------------------------------------
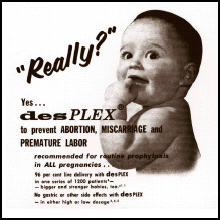 DES (diethylstilbestrol) was one of Canada's worst drug disasters. Between 200,000 and 400,000 pregnant women and their children were unnecessarily exposed to a harmful medicine with tragic results.
DES (diethylstilbestrol) was one of Canada's worst drug disasters. Between 200,000 and 400,000 pregnant women and their children were unnecessarily exposed to a harmful medicine with tragic results.
DES was the first synthetic estrogen. The drug was prescribed to prevent miscarriage between 1941 and 1971 in North America (longer in Europe), but proved ineffective. Although good evidence form animal studies indicated the DES might cause cancer, the drug
- Was prescribed to millions of women worldwide.
- Continued to be used in pregnancy nearly 20 years after it was found to be ineffective.
- Was found to cause cancer in young women in 1971, thirty years after it was first prescribed.
------------------------------------------------------------------
Research and writing:
Barbara Mintzes.
Conception of the series "Protecting Our Health" and revision:
Rosanna Baraldi.
Other titles in this series include:
"Direct-to-consumer Prescription Drug Advertising: When public health is no longer a priority" "Who benefits? International Harmonisation of the Regulation of New Pharmaceutical Drugs" "Preventing Disease: Are Pills the Answer?"
For a bibliography or for more information about Women and Health Protection, visit the website at http://www.whp-apsf.ca or write to: WHP, P.O. Box 291, Station Q, Toronto, Ontario, M4T 2M1
For information on DES, visit DES Action Canada on the web: http://www.web.net/~desact
This project has been made possible with grants from the Centre of Excellence for Women's Health, York University and the Women's Health Bureau, Health Canada. The views expressed herein do not necessarily represent the official policy of Health Canada.
Copyright © DES Action Canada 2000, second edition 2003.
Copyright ©2006-2007 Women and Health Protection

